Navigating the Future: Growth Strategies, Key Players, and Segments in the Cardiac Safety Services Market
Introduction
The cardiac safety services market is expanding at a rapid pace due to the increasing prevalence of cardiovascular disease (CVD), regulation, and increased complexity in clinical trials. Increased R&D expenditure by pharmaceutical and biotech firms is driving demand for comprehensive cardiac safety analysis . This blog discusses the market growth strategies, names key players, and emphasizes top segments which are driving the industry direction.
Market Overview
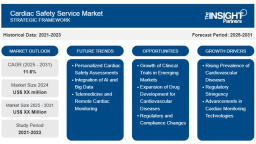
The cardiac safety services market globally was approximately worth, at a compound annual growth rate (CAGR) of 11.6%. The drivers behind the growth include increasing CVDs, the evolution of cardiac monitoring devices, and increased outsourcing of clinical trials to specialized service organizations.
Growth Strategies
The cardiac safety services market is increasing steadily through several strategic drivers. Technological developments, in the guise of the intersection of machine learning and AI, have enhanced the accuracy and efficiency of cardiac data analysis. The technologies, when combined with wearable sensors and remote monitoring, facilitate real-time detection of cardiac anomalies and continuous data capture, which improve patient compliance. Compliance with regulatory guidelines is also among the key drivers, with the FDA and EMA enforcing guidelines requiring extensive cardiac monitoring, compelling pharma firms to collaborate with experienced service providers. Strategic partnerships, mergers, and acquisitions are also driving the market and allowing firms to go deeper and pool resources for improved delivery of services. Clinical trial outsourcing to CROs is also on the increasing trend. The strategy provides access to technology and expertise and helps pharmaceutical companies to become more streamlined, reduce expenditure, and meet the requirements while focusing on core R&D activities. All such strategies collectively drive market growth and change.
Key Market Players
Cardiac safety services are supported by different key players who provide the concerned assistance to pharmaceutical and biotech companies during clinical trials. IQVIA leads with cross-technology and analytics strengths that complement cardiac monitoring services. Charles River Laboratories and Covance (Labcorp Drug Development) are most prized for preclinical and clinical services. Medidata Solutions offers advanced data analytics solutions for cardiac safety data capture and analysis. Parexel International and PRA Health Sciences in the portfolio of ICON plc offer global trial management with utmost focus on regulatory compliance and patient safety. Syneos Health stands out from the rest as it offers end-to-end clinical solutions, while Toxikon focuses on toxicology and safety pharmacology studies. Bio-Optronics, being a clinical trial management systems specialist, also offers cardiac safety programs. These organizations play an important role in driving drug development with the comprehensive cardiac evaluation of investigation therapies.
Key Segments of the Market
By Service Type
ECG/Holter Monitoring: It is the leading market segment because it universally plays a part in clinical research in the detection of arrhythmias and other cardiac events. Continuous monitoring with ECG and Holter devices gives full analysis of the heart
Blood Pressure Monitoring: To measure the hemodynamic effects of study medication, this service identifies potential cardiovascular risk.
Cardiovascular Imaging: Imaging technologies like echocardiography and cardiac MRI provide extensive structural and functional data, supplementing detailed cardiac evaluation.
Extended QT Studies: Extended QT studies assess the drug's capacity to prolong the QT interval, a primary consideration in proarrhythmic risk assessment.
By Type
Integrated Services: Complete packages that include several cardiac safety tests as one package with simplicity of solution for clinical trials.
Standalone Services: Standalone cardiac safety tests designed to address unique trial requirements, offering sponsors flexibility.
By End-User
Pharmaceutical and Biotechnology Companies: Direct end-users of cardiac safety services, relying on these tests to determine drug safety and regulatory approval.
Contract Research Organizations (CROs): Use cardiac safety services to enable outsourced clinical trials, offering specialist skills to sponsors.
Regional Insights
Regional trends and Cardiac Safety Services Market drivers of the forecast period have been clearly described by Insight Partners' researchers. Cardiac Safety Services Market egments and geography in North America, Europe, Asia Pacific, Middle East and Africa, and South and Central America have also been covered here.
Conclusion
The cardiac safety services market will see huge growth fueled by technology, regulatory demands, and increased clinical trial complexity. As the demand for increased cardiac safety assessment increases, service providers will have to innovate and collaborate in an effort to address the evolving needs of the pharmaceutical and biotechnology sectors. By leveraging the application of cutting-edge technologies, regulatory expertise, and adaptive service models, the industry leaders can take advantage of new opportunities and assist in creating safer, more effective therapeutics.




